Table of Contents
Molecular orbitals of Ethene
In this exercise, you will perform an electronic structure calculation to obtain the ethene molecular orbitals (MOs). If performed correctly, your calculations will produce a list of occupied and non occupied MOs and a series of *.cube files, that allow the visualization of the oribital with VMD.
1. Step: Run the calculation
Run a calculation with the following (commented) input file.
Note that the file contains explicit basis sets and potential for all-electron calculations. An explanation of the basis set formats is given here: Basis Sets
- ethene.inp
&GLOBAL PROJECT ethene RUN_TYPE ENERGY PRINT_LEVEL MEDIUM &END GLOBAL &FORCE_EVAL METHOD Quickstep ! Electronic structure method (DFT,...) &DFT &PRINT &MO_CUBES ! Controls which MOs are written to cube-files. NHOMO 5 NLUMO 5 &END MO_CUBES &PDOS ! Controls which MOs are included in the pdos-files. NLUMO 5 &END &END PRINT &POISSON ! Solver requested for non periodic calculations PERIODIC NONE PSOLVER WAVELET ! Type of solver &END POISSON &QS ! Parameters needed to set up the Quickstep framework METHOD GAPW ! Method: gaussian and augmented plane waves &END QS &SCF ! Parameters controlling the convergence of the scf. This section should not be changed. MAX_ITER_LUMOS 10000 EPS_SCF 1.0E-6 SCF_GUESS ATOMIC MAX_SCF 60 EPS_LUMOS 0.000001 &OUTER_SCF EPS_SCF 1.0E-6 MAX_SCF 6 &END &END SCF &XC ! Parametes needed to compute the electronic exchange potential &XC_FUNCTIONAL NONE ! No xc functional &END XC_FUNCTIONAL &HF ! Hartree Fock exchange. In this case is 100% (no fraction specified). &SCREENING ! Screening of the electronic repulsion up to the given threshold. EPS_SCHWARZ 1.0E-10 ! Threshold specification &END SCREENING &END HF &END XC &END DFT &SUBSYS &CELL ABC 10 10 10 PERIODIC NONE ! Non periodic calculations. That's why the POISSON scetion is needed &END CELL &TOPOLOGY ! Section used to center the atomic coordinates in the given box. Useful for big molecules &CENTER_COORDINATES &END &END &COORD C -2.15324 3.98235 0.00126 C -0.83403 4.16252 -0.00140 H -0.25355 3.95641 0.89185 H -0.33362 4.51626 -0.89682 H -2.65364 3.62861 0.89669 H -2.73371 4.18846 -0.89198 &END COORD &KIND H ! Basis set and potential for H &BASIS 2 1 0 0 3 1 18.73113700 0.03349460 2.82539370 0.23472695 0.64012170 0.81375733 1 0 0 1 1 0.16127780 1.00000000 &END POTENTIAL ALL &POTENTIAL 1 0 0 0.20000000 0 &END &END KIND &KIND C ! Basis set and potential for C &BASIS 4 1 0 0 6 1 3047.52490000 0.00183470 457.36951000 0.01403730 103.94869000 0.06884260 29.21015500 0.23218440 9.28666300 0.46794130 3.16392700 0.36231200 1 0 1 3 1 1 7.86827240 -0.11933240 0.06899910 1.88128850 -0.16085420 0.31642400 0.54424930 1.14345640 0.74430830 1 0 1 1 1 1 0.16871440 1.00000000 1.00000000 1 2 2 1 1 0.80000000 1.00000000 &END POTENTIAL ALL &POTENTIAL 4 2 0 0.34883045 0 &END &END KIND &END SUBSYS &END FORCE_EVAL
2. Step
If the calculation was performed correctly, a number of new files should have been written:
$ ls *.pdos *.cube ethene-k1-1.pdos ethene-WFN_00004_1-1_0.cube ethene-WFN_00006_1-1_0.cube ethene-WFN_00008_1-1_0.cube ethene-WFN_00010_1-1_0.cube ethene-WFN_00012_1-1_0.cube ethene-k2-1.pdos ethene-WFN_00005_1-1_0.cube ethene-WFN_00007_1-1_0.cube ethene-WFN_00009_1-1_0.cube ethene-WFN_00011_1-1_0.cube ethene-WFN_00013_1-1_0.cube
First have a look at the *.pdos files. PDOS stands for Projected Density of States. These files list the energies and occupation of the MOs. Furthermore, they show how the MOs are compose from basis-functions of different atoms (one pdos-file for each atomic kind) and angular momentum (s,p,d). Hence, these numbers always sum up to 1.0.
3. Step
Now look at the *.cube files.
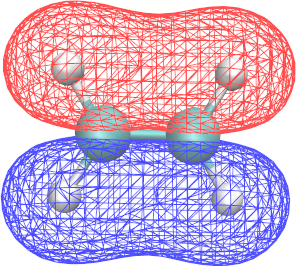
Each cube-file contains the electronic density of one MO mapped onto a regular 3D-grid. Not all MOs were written to a cube-file, this is controlled by the PRINT_MO section. Their filenames tell you to which MO a cube-file belongs. For example ethene-WFN_00005_1-1_0.cube contains the 5th orbital.
Use VMD to visualize the cube-files:
- To run
vmd ethene-WFN_00008_1-1_0.cube - To visualize the molecule (sometimes it's not visible by default):
Graphics > Representations > Draw style > Drawing Method=CPK - Add a second representation by clicking on Create Rep
- In this second representation set Drawing Method=Isosurfaces and Draw=Wireframe
- Finally set the Isovalue of to a reasonable value, eg. 0.1 .
- To visualize the positive and the negative part of an orbital simultaneously, you will have to add a third representation with a negative Isovalue, e.g. -0.1 .
- To give the two representations different colors, set their Coloring Method=ColorID and choose different ids.
Questions
- Quickly sketch the energy distribution of the MOs.
- What's the energy of the HOMO, LUMO, and the band-gap?
- Use VMD to identify the shape and energy of the $\pi$ and $\pi^*$ orbitals.