Table of Contents
Reaction Energy
In this exercise, you will calculate the reaction energy for the methane combustion reaction: \[ CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O \]
Reaction energy: \[ \sum E_\text{products} - \sum E_\text{rectants} = \left (2\cdot E_{H_2O} + E_{CO_2} \right) - \left(E_{CH_4} + 2\cdot E_{O_2}\right) \]
Ground state oxygen, O$_2$, is a triplet diradical, a property which can explain why liquid oxygen is paramagnetic and attracted to the poles of a magnet.
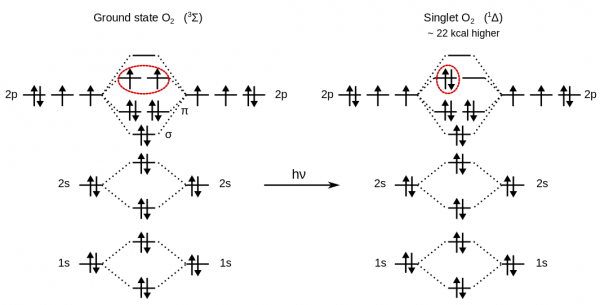
For this reason, to get the energy of the O$_2$ molecule, a LSD calculation is required.
1.Step
Run a single point calculation for CH$_4$, using the given input file. Note that the file contains explicit basis sets and potential for all-electron calculations. An explanation of the basis set formats is given here: Basis Sets
- CH4.inp
&GLOBAL PROJECT CH4 RUN_TYPE ENERGY PRINT_LEVEL MEDIUM &END GLOBAL &FORCE_EVAL METHOD Quickstep ! Electronic structure method (DFT,...) &DFT &POISSON ! Solver requested for non periodic calculations PERIODIC NONE PSOLVER WAVELET ! Type of solver &END POISSON &QS ! Parameters needed to set up the Quickstep framework METHOD GAPW ! Method: gaussian and augmented plane waves &END QS &XC ! Parametes needed to compute the electronic exchange potential &XC_FUNCTIONAL NONE ! No xc functional &END XC_FUNCTIONAL &HF ! Hartree Fock exchange. In this case is 100% (no fraction specified). &SCREENING ! Screening of the electronic repulsion up to the given threshold. EPS_SCHWARZ 1.0E-10 ! Threshold specification &END SCREENING &END HF &END XC &END DFT &SUBSYS &CELL ABC 10 10 10 PERIODIC NONE ! Non periodic calculations. That's why the POISSON section is needed &END CELL &TOPOLOGY ! Section used to center the atomic coordinates in the given box. Useful for big molecules &CENTER_COORDINATES &END &END &COORD C 4.6425962273 5.0574874650 5.2069537560 H 5.7240587065 5.0555482951 5.2189766147 H 4.2766068912 5.8773176685 5.8100567767 H 4.2759350196 4.1226994019 5.6087492584 H 4.2938562590 5.1744089096 4.1899119266 &END COORD &KIND H ! potential and basis for H &BASIS 3 1 0 0 3 1 12.25200000 0.02282200 1.86870000 0.15564000 0.41821000 0.48898000 1 0 0 1 1 0.10610000 1.00000000 1 1 1 1 1 1.00000000 1.00000000 &END POTENTIAL ALL &POTENTIAL 1 0 0 0.20000000 0 &END &END KIND &KIND C ! potential and basis for C &BASIS 5 1 0 0 6 2 1252.60000000 0.00557360 0.00000000 188.57000000 0.04149600 -0.00027440 42.83900000 0.18263000 -0.00255830 11.81800000 0.46129000 -0.03337500 3.55670000 0.44931000 -0.08730500 0.54258000 0.00000000 0.53415000 1 0 0 1 1 0.16058000 1.00000000 1 1 1 3 1 9.14260000 0.04449900 1.92980000 0.23108000 0.52522000 0.51227000 1 1 1 1 1 0.13608000 1.00000000 1 2 2 1 1 0.80000000 1.00000000 &END POTENTIAL ALL &POTENTIAL 4 2 0 0.34883045 0 &END &END KIND &END SUBSYS &END FORCE_EVAL
If the calculation was performed correctly, the total energy of the CH$_4$ molecule is printed in the output file.
**** **** ****** ** PROGRAM STARTED AT ***** ** *** *** ** PROGRAM STARTED ON ** **** ****** PROGRAM STARTED BY ***** ** ** ** ** PROGRAM PROCESS ID **** ** ******* ** PROGRAM STARTED IN ..... ENERGY| Total FORCE_EVAL ( QS ) energy (a.u.): ..... **** **** ****** ** PROGRAM ENDED AT ***** ** *** *** ** PROGRAM RAN ON ** **** ****** PROGRAM RAN BY ***** ** ** ** ** PROGRAM PROCESS ID **** ** ******* ** PROGRAM STOPPED IN
2.Step
Modify the input in order to perform the same calculation for:
- H$_2$O
- CO$_2$
- O$_2$ triplet
For O2 triplet, the LSD and MULTIPLICITY keywords are needed in the DFT section:
METHOD Quickstep
&DFT
LSD ! Requests a spin-polarized calculation for unpaired electrons
MULTIPLICITY 3 ! Multiplicity = 2S+1 (S= total spin momentum)
...
Another example can be found here Basis Sets
3.Step
At the end, you should get a table like:
| Species | Total Energy |
|---|---|
| CH$_4$ | … |
| O$_2$ | … |
| H$_2$O | … |
| CO$_2$ | … |
Now you can compute the overall reaction energy.
Questions
- What are the total energies of O$_2$, H$_2$O, CO$_2$, and CH$_4$?
- What is the overall reaction energy of the CH$_4$ combustion?
- (Optional) What is the total energy difference between the O$_2$ singlet and triplet state?
Appendix
Basis Set for Oxygen
#O pc-1
5
1 0 0 6 2
2306.70000000 0.00539400 0.00000000
347.15000000 0.04024800 -0.00031692
78.89000000 0.17921000 -0.00259440
21.87600000 0.45978000 -0.03624100
6.66460000 0.45234000 -0.08779000
1.06690000 0.00000000 0.53320000
1 0 0 1 1
0.30700000 1.00000000
1 1 1 3 1
17.02200000 0.04891900
3.68380000 0.24962000
0.99234000 0.51347000
1 1 1 1 1
0.24487000 1.00000000
1 2 2 1 1
1.00000000 1.00000000
Potential for Oxygen
#O ALLELECTRON ALL
4 4 0
0.24762086 0
Coordinates for O$_2$
O 4.4720538104 4.7584649515 4.9999999998 O 5.5279461896 5.2415350485 4.9999999995
Coordinates for CO$_2$
C 4.9999776408 4.9999662056 4.9999894728 O 5.6486993295 5.9339540261 5.0004691016 O 4.3512530072 4.0659797648 4.9995464311
Coordinates for H$_2$O
O 4.6926974603 4.7525411835 4.6307067609 H 5.6350172910 4.8022721035 4.7052454388 H 4.3528571397 5.2445222023 5.3644975249
